Dopaminergic System
Projects in our laboratory are focused on the identification of novel mechanisms involved in regulation of dopamine neurotransmission in the brain. In particular we are interested in the regulation of dopamine transporter (DAT) an integral membrane protein vital for regulation of synaptic dopamine. Work in our laboratory is devoted to understanding the details of DAT regulation, trafficking, the pharmacological modulation of the transporter and how this is all integrated into the broader context of normal dopamine signaling and the pathogenesis of addiction, neurological and neuropsychiatric disorders.
Psychostimulants regulation of DAT activity
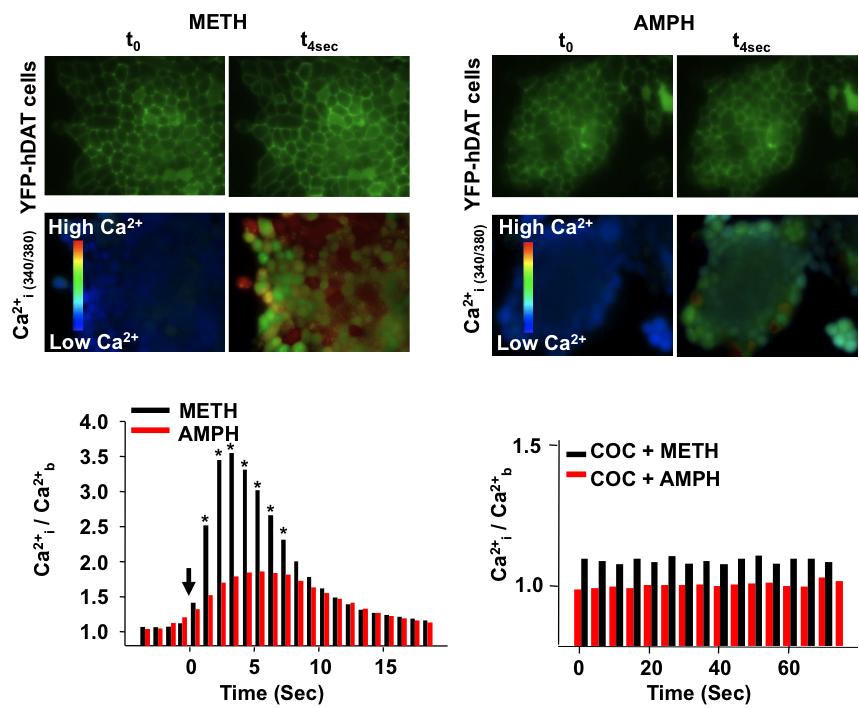
The psychostimulants d-amphetamine (AMPH) and methamphetamine (METH) release excess dopamine (DA) into the synaptic clefts of dopaminergic neurons. Abnormal DA release is thought to occur by reverse transport through the DA transporter (DAT), and it is believed to underlie the severe behavioral effects of these drugs. Here we compare structurally similar AMPH and METH on DAT function in a heterologous expression system and in an animal model. In the in vitro expression system, DAT-mediated whole-cell currents were greater for METH stimulation than for AMPH. At the same voltage and concentration, METH released five times more DA than AMPH and did so at physiological membrane potentials. At maximally effective concentrations, METH released twice as much [Ca(2+)](i) from internal stores compared with AMPH. [Ca(2+)](i) responses to both drugs were independent of membrane voltage but inhibited by DAT antagonists. Intact phosphorylation sites in the N-terminal domain of DAT were required for the AMPH- and METH-induced increase in [Ca(2+)](i) and for the enhanced effects of METH on x[Ca(2+)](i) elevation. xCalmodulin-dependent protein kinase II and protein kinase C inhibitors alone or in combination also blocked AMPH- or METH-induced Ca(2+) responses. Finally, in the rat nucleus accumbens, in vivo voltammetry showed that systemic application of METH inhibited DAT-mediated DA clearance more efficiently than AMPH, resulting in excess external DA. Together these data demonstrate that METH has a stronger effect on DAT-mediated cell physiology than AMPH, which may contribute to the euphoric and addictive properties of METH compared with AMPH.
Partner proteins regulation of DAT function
1. α-synuclein regulation of dopamine transporter
Recent work in various disease models has begun to emphasize the significance of presynaptic dysfunction as an early event that occurs before manifestation of neurological disorders. An increasing number of proteins have been identified that interact with different domains of DAT and regulate the biology of the transporter. These interactions suggest that the synaptic distribution, targeting, compartmentalization, trafficking and functional properties of DAT can be regulated via interacting proteins. α-Synuclein is a 140-amino acid protein that forms a stable complex with DAT and is linked to the pathogenesis of neurodegenerative disease. To elucidate the potential functional consequences of DAT/α-synuclein interaction, we explored α-synuclein modulation of DAT activity in midbrain dopaminergic neurons obtained from TH::RFP mice, immortalized DA neurons, and a heterologous system expressing DAT. We used dual pipette whole cell patch clamp recording to measure the DAT-mediated current before and after dialysis of recombinant α-synuclein into immortalized DA neurons. Our data suggest that intracellular α-synuclein induces a Na+ independent but Cl--sensitive inward current in DAT-expressing cells. This current is blocked by DAT blocker GBR12935 and is absent when heat-inactivated α-synuclein is dialyzed into these cells. The functional consequence of this interaction on DAT activity was further examined with real-time monitoring of transport function using a fluorescent substrate of DAT, 4-(4-(dimethylamino)styryl)-N-methylpyridinium (ASP+). Overexpression of α-synuclein in DAT-positive immortalized DA neurons and CHO cells expressing DAT decreased the magnitude and rate of DAT-mediated substrate uptake without a decrease in the initial binding of the substrate at the plasma membrane. Taken together our findings are consistent with the interpretation that DAT/α-synuclein interaction at the cell surface results in a DAT-dependent, Na+-insensitive, Cl-sensitive inward current with a decrease in substrate uptake, suggesting that DAT/α-synuclein interaction can modulate dopamine transmission and thus neuronal function.
2. Sigma-1R regulation of Dopamine transporter
Methamphetamine abuse is a major public health problem with no effective treatment strategies or FDA-approved pharmacotherapies. Methamphetamine addiction is thought to be a chronic disease rooted in numerous neurobiological adaptations induced by this psychostimulant. Arguably, the greatest promise for the treatment of methamphetamine abuse lies in determining the underlying molecular mechanisms of these neuronal adaptations to generate the opportunity of developing targeted therapies. The long-term goal of this work is to identify these underlying molecular mechanisms. The dopamine transporter (DAT) is the primary target for psychostimulants such as methamphetamine and cocaine. By inhibiting dopamine transporter uptake, these drugs are able to increase extracellular dopamine concentration, which modulates dopamine-associated behaviors. However, what is not often recognized is that methamphetamine is a substrate for the dopamine transporter and a high affinity ligand at the sigma-1 receptor (sigma1R), an intracellular chaperon protein. Our preliminary data suggest that: 1) the sigma1R is expressed in the soma and dendrites of midbrain dopaminergic neurons; 2) the sigma1R is co-localized and interacts with the DAT; 3) the administration of either a sigma1R agonist or methamphetamine increases the co-localization and interaction of DAT/sigma1R; and 4) co-administration of a sigma1R agonist influences methamphetamine-regulation of dopamine uptake, dopamine efflux, and the excitability of dopaminergic neurons. We will test the overarching hypothesis that methamphetamine regulation of dopamine transporter function relies in part on methamphetamine-induced increases in sigma1R/DAT interactions and their functional consequences. We will focus on determining the mechanism of DAT/sigma1R interaction (how), the cellular localization of DAT/sigma1R interaction (where), and the functional consequence of this interaction (what). If, as we postulate, the interaction with the sigma1R represents a main intracellular mechanism for mediating methamphetamine-regulation of DAT, then we will have identified a possible target for treating methamphetamine addiction and other psychiatric diseases involving dysregulation of dopamine neurotransmission.
Membrane localization of dopamine transporter
Though the trafficking of DAT to and away from the surface membrane has been studied extensively, it is unknown whether or not changes in the membrane potential also can regulate the trafficking of DAT. Our simultaneous patch-clamp and Total Internal Reflection Fluorescence (TIRF) confocal microscopy suggest that DAT changes in the membrane potential regulates the trafficking of Dopamine transporter. We found that membrane hyperpolarization increases YFP-DAT level at the membrane. The hyperpolarization-induced increase in surface DAT level is paralleled by an increase in DAT-mediated inward current. Currently we are examining the hypothesis that changes in the membrane potential regulate the trafficking of DAT to and from the cell surface membrane in the DA neurons.
The communication between dopamine neurons and microglia is bidirectional.
Microglia modulate neurotransmission, facilitate synapse formation and dissolution, and provide neuronal protection, but cellular/molecular mechanisms are incompletely understood. Much of the premise for interactions between dopamine neurons and microglia is supported by presence of dopamine receptors on microglial cells allowing them to respond to neuronal signals. The idea is that dopamine receptor stimulation on microglial cells alters microglial function, which in turn could then (reciprocally) affect dopamine neurotransmission. We will examine how microglial activity is affected by dopaminergic signaling, and conversely how microglial products may modulate dopamine neurotransmission. This work will address a significant knowledge gaps in the field revealing the cellular/molecular mechanisms underlying bidirectional communication between dopamine neurons and microglia,